Toxicological Analysis of Chemicals Used at
PACIFIC STEEL CASTING COMPANY
Toxicological Analysis of Chemicals Used at PACIFIC STEEL CASTING COMPANY
Selina Bendix, Ph.D. R.E.A.
Prepared for Department of Health & Human Services City of Berkeley
9 November 1990SUMMARY
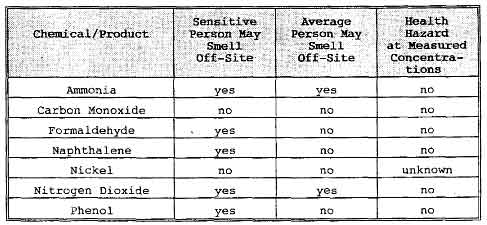
Community concern has been expressed about the potential health hazard of odors detected by residents near Pacific Steel Casting Company (PSC) in Berkeley, California. A study has been made of chemicals used and produced at PSC, their odor characteristics, and the health hazards associated with known or probable concentrations of these chemicals. Information about chemicals of health concern is summarized in the table below.
Summary of Properties of Chemicals Used/Produced at Pacific Steel Casting Company
No health hazards were found. A number of chemicals are sometimes present at levels which some or most people can smell. Recommendations are made for chemical analyses to be performed to determine whether there may be any health risks for certain chemicals for which no analytical data is available.
INTRODUCTION
In order to evaluate the potential health hazards of emissions from Pacific Steel Casting Company (PSC), a study has been made of the MSDSs (Material Safety DataSheets)1 for all materials used at Pacific Steel Casting (two 3-inch binders). Chemical air analysis data from three studies performed by consulting firms for PSC has been considered in this evaluation: 1989 stack emission data from Aqua Terra2 submitted to the Bay Area Air Quality Management District (BAAQMD), 1989 ENSR data from an area downwind of PSC where odors had been reported,3 and 1988 Clayton data.4
An independent evaluation has been made of the toxicological properties of the materials contained in the products in use at the site. Where judged appropriate, computer search of the latest version of the National Institute of Occupational Safety and Health (NIOSH) Registry of Toxic Effects of Chemical Substances (RTECS) as been performed. Such information is identified as coming from RTECS On-Line, 1990."
In view of neighborhood complaints of odors emanating from the site, the odor thresholds for chemicals in use at the site have been considered, but odor has not been used as a criterion for health concern since odorless substances may have significant toxicological effects.
This report focuses on major production chemicals, since small volume chemicals used in maintenance were generally considered to have little potential for dispersion off-site or for analytical or odor detection off-site. A site tour on 23 July 1990 was made to determine typical operation practices at the site. Several meetings at the site offered additional opportunities to observe some of the production activities and smell odors at the site. Some odors were detected within the facility outdoors and inside production buildings. The odors varied with location and time of day, appearing to be strongest in the 7 to 9 am period. None of the odors were recognizable as characteristic of specific chemicals known to be used or produced at the site.
During the tour, safety procedures were observed to be followed, and employees made use of protective equipment. Safety practices were in use in portions of the facility not originally on the expected tour route and the apparent ease of movement of workers using safety equipment indicated that this was routine operation and not a show put on for observers.
Some production materials used on the site either are relatively nontoxic, nonvolatile,5 or non-powdery solids which would not be dispersed away from the area where they are used and would not constitute an environmental or health hazard if occasional dispersion should occur. These materials are generally not specifically covered in this report which is focused on findings regarding potentially toxic materials which could be dispersed off-site.
PRODUCTS USED AT THE SITE AND THEIR CONSTITUENT CHEMICALS
Acme Plasti-Sand (712E0-2b6)
This large-volume production product is used as glue for sand molds into which molten metal is poured.
This product contains Novolak7 resin, hexamethylenetetramine, silica sand, and less than 1% of phenol. The resin coating on this silica sand would coat small particles to make them larger and cause them to stick together and thus tend to prevent dispersion of fine silica dust which is toxic to the lungs. The coating on the particles would prevent the silica from coming into contact with tissue in the lung if any particles were inhaled. The MSDS indicates that the material is a skin sensitizer. Hexamethylenetetramine is a mutagen8 in 9 tests,9 an eye irritant, and both skin contact and inhalation of fumes can result in skin rashes.10,11 Common methods of air analysis do not detect hexamethylenetetramine. PSC workers use respirators and gloves to protect them from exposure to this material.
Ammonia, carbon monoxide, formaldehyde, nitrogen oxides and phenol are released when the plastic sand binder is broken down by heat as molten metal is poured into the sand molds. Polyaromatic hydrocarbons (PAHs) are probably formed in the hottest portion of the mold next to the metal.12 Ventilation should be good to prevent irritation from inhalation of ammonia or nitrogen oxides, sensitization by formaldehyde, or decreased alertness due to inhalation of carbon monoxide. The ventilation instructions on the MSDS should be followed.
No odor of ammonia was detected during any visit to the site. No ammonia was detected in the air sample of air downwind (all measurements identified as "downwind" were made in the Cal Ink parking 1t at 1404 Fourth Street, east of PSC where there are no buildings between PSC and the nursery school site across the street) from the site or in the Clayton air samples east (in the same Cal Ink parking lot) and west of PSC (across the frontage road from PSC, towards Interstate 80). The analytical detection levels (ENSR 2.1 parts per million, ppm; Clayton 0.4 ppm) were not as sensitive as some human noses, but the higher of the detection levels was about one tenth of the lowest-concentration known to be irritant to humans, see Table 1.
There could be enough ammonia to be smelled off-site, but there would not be enough ammonia to be irritating or to cause toxic effects.
Formaldehyde is an IARC (International Agency for Research on Cancer) carcinogen and a strong sensitizing agent. Sensitizing agents cause allergic responses such as asthma and skin rashes. See Table 2 for information about formaldehyde.
There was no odor of formaldehyde present at any time the site was visited. (If you have dissected animals in a strong smelling preservative, they were probably preserved in formaldehyde.)
Typical indoor concentrations of formaldehyde range from 0.02 to 0.5 ppm. These concentrations are often in excess of the California Department of Health Services recommendation for a maximum of 0.05 ppm.13
The national average outdoor formaldehyde concentration is below 0.016 ppm. The EPA recommended outdoor standard is 0.1 ppm.14 The maximum concentration of formaldehyde found by ENSR downwind from PSC of 0.07 ppm was high enough to be smelled by sensitive persons. It was below the concentration causing eye irritation, below the EPA recommended outdoor air standard, and within the range of California indoor formaldehyde concentrations. It was more than ten times less than the lowest concentration which causes peoples' eyes to tear. Formaldehyde was not detectable in 1988 measurements made by Clayton with a method sensitive to 0.1 ppm of formaldehyde15
Stack emissions were sampled for formaldehyde analyses during casting operations which would release formaldehyde. Three stacks receive air from areas where formaldehyde is released. Three samples were taken for chemical analysis from each of these three stacks. These stacks are 30, 40 and 85 feet tall.
The formaldehyde values for the three stacks were: undetectable (less than 1.5 ppm16), 0.6 to 3.6 ppm, and 0.5 to 1.1 ppm. The total of the average emissions of these three stacks was 2.4 ppm. These stack emissions are diluted by clean air as they move away from the top of the stack. Generally they will be diluted below the odor threshold before they reach the perimeter of the site, but occasionally odor may be detectable to sensitive persons. Concentrations high enough to produce immediate symptoms would not occur.
The maximum lifetime expected excess cancer risk from worker 8-hour/day exposure to 0.1 ppm of formaldehyde is 1 cancer death in ten million persons exposed17 Multiplying this by three to get the nonoccupational risk for similar persons exposed 24-hours per day for a 70 year lifetime, the risk is about 3 cancer deaths per ten million exposed persons. The 8-hour risk front the maximum formaldehyde level of 0.07 ppm found downwind of PSC (the two other ENSR samples had o detectable formaldehyde with a detection threshold of 0.03 ppm18) would be about 0.7 cancer deaths per ten million persons exposed, and the 24-hour risk would be about 2 cancer deaths per ten million persons exposed. Since formaldehyde is a direct carcinogen which does not require chemical activation in the body in order to form the actual carcinogenic molecule, no adjustment of this risk estimate for children or the elderly is required.19 The actual average formaldehyde level is an unknown amount less that 0.07 ppm, since two samples out of three had no detectable formaldehyde, or less than 0.03 ppm.
There was no odor of nitrogen oxides at the time of any visit to the PSC site. The average human odor detection level20 for nitrogen dioxide, the commonest nitrogen oxide, is 0.4 ppm.21 Typical indoor concentrations of nitrogen dioxide range from 0.004 to 05 ppm with 1.0 ppm peaks22 The average one-hour state standard for nitrogen dioxide is 0.25 ppm and the average annual federal standard for this substance is 0.05 ppm.23 Most of the nitrogen dioxide in the air comes from automobile exhaust.
Nitrogen dioxide was not detected downwind of the site by a method with a detection threshold of 1.25 ppm,24 which is higher than the odor detection level and lower than the maximum recommended occupational exposure level of 3 ppm.25 Clayton found 0.2 ppm of nitrogen dioxide east and west of PSC. The lowest concentration of nitrogen dioxide which can damage human lungs in a 10 to 40 minute exposure is 6 to 90 ppm.26 Since the maximum amount of nitrogen dioxide present was 0.2 ppm, no lung effects would have resulted allowing for a ten times lower effect threshold for sensitive persons exposed for up to two hours, or 0.6 ppm,27 but there could have been enough present to smell. Clayton found less than 0.1 ppm of another nitrogen compound, nitric oxide, east and west of PSC.
Carbon monoxide (an odorless gas) was not detected by ENSR downwind from PSC by a method with a detection threshold of 8.3 ppm. Clayton did not detect carbon monoxide east or west of PSC with a method sensitive to 5 ppm of carbon monoxide. Typical indoor concentrations of carbon monoxide range from 4 to 40 ppm.
A ninety minute exposure to 50 ppm of carbon monoxide affects judgment of time intervals, four hours exposure to 200 ppm causes a mild headache; and 2.5 hours exposure to 1000 ppm causes a severe headache and decreased manual dexterity.28 There was not enough carbon monoxide present to cause detectable symptoms.No odor of phenol 29 was detected outdoors or in the casting area of the plant at the time of tour on 23 July 1990. Phenol in stack emissions during operations which would release phenol was below the detection level of 0.001 ppm in all but one sample from one stack which was 0.004 ppm. Phenol is carcinogenic30 and neurotoxic.
The stack emissions are generally more than ten times less than the detection threshold for a sensitive person, see Table 3, to the right. Downwind of PSC the concentration of phenol is above the detection threshold for sensitive people and below the average odor detection level. Phenol sources other than PSC may be contributing to this phenol level since the level is higher than the PSC stack emissions and the odor of phenol is not noticeable on the site.
The 0.06 ppm phenol level downwind of PSC is about 1% of the maximum permitted occupational exposure level for 40 hours a week and about 0.1% of the lowest concentration of phenol known to harm mammals. Allowing for the fact that infants and the elderly may be ten times more sensitive than the average working adult to this well-studied compound, no human health effects would be caused at this level.
Gasoline Chevron Regular
The MSDS for this gasoline indicates that it contains up to 4.95% benzene.31 Benzene is rated by IARC as a human-positive carcinogen. This gasoline also contains less than 0.1% each of ethylene dibromide32 (the pesticide, also a common gasoline additive) and ethylene dichloride, both IARC animal-positive carcinogens.
Benzene causes leukemia and lymphoma in people and these cancers as well as endocrine gland and lung cancers in animals. Benzene is a mutagen in 62 tests, including 9 tests involving human inhalation or human cells. Benzene is a teratogen33 causing musculoskeletal defects and affecting embryonic bone marrow in animals. Ethylene dibromide is a powerful mutagen, decreases sperm formation, and causes cancer of blood vessels, lungs, gastrointestinal tract, liver, and skin in animals. Any chemical which is carcinogenic in animals can be presumed to be a human carcinogen34 Ethylene dichloride is a powerful mutagen and causes cancer of the skin, lungs, blood vessels, gastrointestinal tract and uterus in animals.
The pronounced toxicity of components of gasoline which is handled routinely in our society with minimal precautions should be taken into consideration in evaluation of the toxicity of other products discussed in this report.
High Alumina Mortar
This product was formerly used for repair of the mortar between refractory bricks in the heat treating furnaces. For the past approximately four years, repair of this mortar has been performed by contractors instead of PSC staff. None of the material remains in storage at PSC. Some of the old mortar presumably remains between the bricks. The product contains quartz, Cristobalite, Tridymite, amorphous silica, coal tar products (asphalt), petroleum pitch, phosphoric acid, lime, and sodium silicate.
Crystobalite, tridymite, amorphous silica, coal tar products and petroleum pitch are carcinogens. Crystobalite is an IARC animal-positive carcinogen which causes lymphoma. Tridymite is an IARC animal positive carcinogen. Amorphous or fused silica is an IARC human probable carcinogen.35
Asphalt consists of asphaltenes, resins, and oils36 made up of saturated and unsaturated hydrocarbons.37 Petroleum pitch is asphalt cut-back which is carcinogenic by the criteria of RIECS, causing cancer of the lungs and skin. Asphalt products contain PAHs which can cause cancer at a variety of sites.
This mortar product would have dispersed as particulates, if at all. The presence of two types of asphalt would make the material so sticky that not much dispersion of particulates would be expected unless the material is heated. Since it was heated every work day when the furnaces were heated, presumably some PAHs were emitted on a regular basis. The past risk from inhaling particulates blown from the site cannot be judged in the absence of information about their past PAH content.
Hot asphalt has a distinct odor which is unpleasant to many people. It is not known what combination of chemicals is responsible for the odor of asphalt-38 If this odor was detected off-site in the past, it would be very likely to arouse complaints even if the concentration were not high enough to identify the odor. This furnace mortar may have been responsible for odor complaints in the early 1980s.
This mortar was replaced about four years ago by Air Setting Fireclay Mortar which contains less than 35% crystalline silica, hydrous alumina silicate, alumina silicate, alumina,, and sodium silicate. Silica is an IARC carcinogen. When used in mortar, the silica is bound in the matrix of the mortar and does not disperse as dust particles which could be inhaled. This material is applied twice a year to replace the existing mortar. This product is safer than the previous mortar and! would not create an odor when it is heated.
Houghto-N-Quench K Oil
This production chemical is a mixture of mineral oils which are IARC human carcinogens, contrary to the MSDS which states that no IARC carcinogens are present. The MSUS is dated 1987 and I suggest that an update be requested from the manufacturer. Less than 100 gallons of this material are used per year.
Since this product is labelled as a quenching oil,39 I assume that it is significantly heated when it is used and that it may be volatilized into the air, and mists of fine droplets may form. Persons working around this material wear respiratory protection and gloves which protects them from the hazards of inhaling mist or volatilized oil and of skin contact.
Total hydrocarbons were nondetectable downwind from the site using a method with a detection threshold of 1 ppm. This suggests that the total organic chemical concentration in the air was low. However, the Clayton report does not contain enough information to make clear whether the method was sensitive to higher molecular weight compounds, such as some of the asphaltenes, so I cannot make a definite interpretation of the meaning of these values.
Houphto-N-Safe 620
This production chemical is a hydraulic fluid that contains 36% ethylene glycol and less than 1% diethylethanolamine. Ethylene glycol (a common antifreeze ingredient) is mutagenic to human white blood cells; is a teratogen at high doses (above 10 grams or about a tablespoonful per kilogram of animal body weight) 1 causing birth defects that affect the musculoskeletal system and craniofacial development on ingestion; is neurotoxic, causing nausea and vomiting when ingested; affects the human liver; and causes tearing of the eyes at high concentrations (3,900 ppm).40 The maximum permitted occupational exposure level is 50 ppm.
Diethylethanolamine is neurotoxic: it affects brain electrical activity, causes convulsions or changes seizure threshold in animals at 1,200 ppm and causes nausea or vomiting when inhaled by humans at 200 ppm.41 Sensitive persons can smell the ammoniacal odor of diethanolamine at 0.01 ppm; the average persons smells it at 0.4 ppm. The odor is usually considered to be unpleasant. The MSDS indicates a possibility of kidney damage from overexposure. The occupational standard is 10 ppm. Analyses were not performed for either of these chemicals. Since there is less than 1% of diethanolarnine in this product, it is unlikely that a concentration high enough tobetoxic ortobesmelled would occur off-site. Unless an ammonia-like odor is detected, there is no risk of health effects from this substance.
These two chemicals are considered safe in common use because relatively high concentrations are required for human toxicological effects. In view of the high concentrations required for adverse effects, it appears unlikely that toxic concentrations of these chemicals would be found on- or off-site.
Inswool Blanket
Inswool blankets are used to cover heated ladles so they do not cool off before molten metal is poured into them. As indicated on the MSDS, the ceramic fiber contained in this product is an IARC carcinogen. The blankets do not tend to shred and free fibers are not released into the air by this use. No off-site exposure to fibers from these blankets would occur.
Line and Pump Solvent
This production product is an aromatic petroleum distillate which contains 11 to 20% naphthalene. Aromatic petroleum distillate is a powerful mutagen.
Naphthalene has an odor (smell of old fashioned moth balls) recognizable by some people at a concentration of 0.0006 ppm (0.6 parts per billion, ppb); however, on the average people smell it at a concentration of 0.1 ppm.42 See Table 4 for information about the relation of the odor threshold of naphthalene to toxic effects of this compound.
No chemical analysis has been performed for the presence of naphthalene in air at the site. Since no one, to my knowledge, reports recognizing the distinctive odor of naphthalene, I think it unlikely that more than 0.1 ppm of naphthalene is present in the air near the site. At 0.1 ppm, an adult would have to breathe the air for 58 days and absorb 100% of the naphthalene in the air to be exposed to the amount of naphthalene required to produce a detectable effect in an oral dose.43 44 No detectable effect would be expected under these circumstances.45
Since the odor of naphthalene may be masked by the presence of other odors, it is possible that as much as 1 ppm could be present without identification of the odor. Under these circumstances, it would take about six days to absorb the single minimum dose to produce symptoms, and no detectable response would be expected.
We have obtained an updated MSDS for this product which will be provided to P5G. Users should be familiar with the symptoms of overexposure given in the MSDS. I note that the updated MSDS correctly indicates that the product may contain chemicals which are carcinogenic.
Magma Quartz Aggregate
This maintenance product aggregate contains silicon dioxide, titanium dioxide, an epoxy diluent, and a Bisphenol A based epoxy resin. About 100 pounds of this cement patch are used per year.
Some forms of silicon dioxide are carcinogenic if inhaled as fine powder. In the presence of epoxy resin, fine powder would not be expected to be present, because the resin would bind fine particles together to a size which would not penetrate the lungs far enough to be carcinogenic. Titanium dioxide is relatively nontoxic. The epoxy diluent is not listed in RTECS On-Line, which indicates either that it is relatively nontoxic or that information about its toxicity is not available in the open literature. The epoxy resin is a powerful mutagen. As indicated on the MSDS, the product is a sensitizer, as expected, since it contains an epoxy resin and epoxies are generally sensitizers.46
If used in accordance with instructions on the MSDS, the product should not be hazardous to workers, and there is no reason why any of its components should be present in the air in sufficient amounts to be detected off-site unless the material happens to be used near the property line. The latter case would be an isolated event for several hours as the cement sets. Since, according to management, there have been no cases of sensitization of workers at the facility (who would be exposed to higher concentrations of vapors from this material than would be present at the perimeter of the site), it is unlikely that enough would be present to sensitize anyone on the other side of the property line. PSC is seeking a less toxic substitute for this material.
Molybdenum Trioxide Amax (briquettes).
This production chemical contains 11% pitch (coal tar pitch volatiles) which is an IARC human-positive carcinogen, causing lung and skin cancers. The carcinogenicity of this material is probably due to its content of PAHs. This product is added to some steels to increase their molybdenum content in order to create a steel with particular properties.47 The sticky pitch in the product will prevent the 4.5% silica from dispersing as dust and contributing to lung damage. The high temperature of the molten steel will completely destroy the PAHs in the pitch and is too high for any organic molecules to survive. The MSDS is generally accurate; although it does not indicate the IARC determination that the material is a human carcinogen. This determination was made in 1987, probably after the preparation of the MSDS in 1987. An updated MSDS should be available from the manufacturer.
Nickel
Amex nickel powder or briquettes is a production chemical which could appear in stack emissions. As indicated in the MSDS, “Nickel fumes are respiratory irritants and may cause pneumonitis. Skin contact may cause an allergic skin rash." Nickel and its compounds are IARC human carcinogens. No nickel was found in the air downwind of PSC with a detection threshold of 0.00014 ppm.48 Nickel was analyzed in PSC stack emissions receiving air from the metals finishing room which was expected to be the highest potential nickel source. Nickel was not detectable by a method sensitive to 0.2 parts per billion (0.0002 ppm). A lifetime exposure to 0.005 parts per billion carries a risk of one cancer in one million exposed persons.49 Since the actual concentration of nickel in the air near PSC is not known and sensitive chemical analyses have already been performed, it is not possible to judge whether enough nickel is present to constitute a greater than one in a million cancer risk for the neighbors of the plant.
Parting Compound-FR-32.
About 13 gallons of this material are used a month as a release agent to ensure that sand molds can be detached from their patterns. This product contains polydimethylsiloxane and methylene chloride.
Some polydimethylsiloxanes are teratogenic. They form gels when the solvent evaporates and would not tend to stay in the air. Methylene chloride is an IARC animal-positive carcinogen; is a teratogen that causes defects in the musculoskeletal system; is a mutagen in human celIs in culture; and causes adverse effects on the nervous system, including causing convulsions or effects on the seizure threshold in humans.50 Since this product is sprayed, the volatile methylene chloride will be widely dispersed.
No measurements of air levels of methylene chloride have been wade on or near the site. Methylene chloride has a "sweetish"51 odor which is usually not found to be unpleasant. The odor is not strong, with sensitive persons detecting it at 0.8 ppm and the average person smelling it at about 30 ppm. Since the odors detected off-site are not described as sweet and have been found to be unpleasant, methylene chloride is probably not contributing significantly to odors off-site. The odor of small amounts of methylene chloride could be masked by other, stronger odors, such as asphalt or formaldehyde. Because of the toxicity of methylene chloride, measurements of methylene chloride should be made.
PEP Set I 53-914
This production chemical is used as a sand binder. It contains 7% phenol and about 50% phenol formaldehyde resin. This material releases phenol and formaldehyde when molten metal is poured into sand molds. See earlier discussion on formaldehyde and phenol on pages 6 and 9, respectively.
Resin, Powdered 5530
This large-volume production material is used as glue for casting molds. It contains less than 0.1% formaldehyde, up to 6.8% phenol, and up to 11% hexamethylenetetramine. When metal is poured into molds sealed by this material, it is heated and some formaldehyde, phenol and hexamethylenetetramine are emitted. See Table 2 for information about formaldehyde. See Table 3 for information about the characteristics of phenol.
SKD-NF Developer
Although less than 10 pounds per year are used of this production chemical for casting inspection, it is a carbon dioxide and dichlorornethane aerosol which will probably be restricted in production to protect the ozone layer. Dichloromethane is an IARC animal-positive carcinogen, a mutagen which inhibits DNA function in human white blood cells, and a teratogen which induces musculoskeletal birth defects.52
Since it is used in small amounts, this product is only a hazard for the person who applies it and finds it difficult to avoid the aerosol vapors. This product would not be detectable off-site because of dilution in a large volume of air. PSC should plan to replace this product to protect its workers and because it will probably not be available in the future. Note that the MSDS calls for use of this material in a spray booth with adequate ventilation.
Stelogen. #2
This and other similar products used for special types of castings which are not routinely made contain calcium fluoride in the form of a powder. Calcium fluoride is irritating to the lungs and eyes. Inhaled fluoride is not known to induce immunological responses and it is not carcinogenic. As indicated in the MSDS, it has no odor. Less than 1000 pounds a year are used of this product.
Particulate emissions have not been analyzed for fluoride and no estimate of potential fluoride emissions has been required by BAAQMD. I cannot judge the probability of irritant concentrations of fluoride being present in the emitted particulates from PSC in the absence of analyses of fluoride in emitted particulates and correlation with neighborhood perception of symptoms. Fluorides would not contribute to odors.
Safety Solvent
This maintenance product consists of 80% trichloroethane (TCA) and 20% of Freon 12 (dichlorodifluoromethane). This is the type of product that is being phased out under the Montreal Protocol to protect the ozone layer in the atmosphere from chlorinated chemicals. There is debate about the carcinogenicity of TCA, but it would be prudent to avoid human exposure since it may be proven to be a carcinogen.
A number of other maintenance products used at the site contain significant amounts of TCA (such as Cleaner/Degreaser, 100% TCA, and Fel Pro Aerosol C-5A, 60% TCA). I doubt that maintenance materials are used in sufficient quantity to be detectable off site, but I cannot be sure in the absence of any analyses for TCA. PSC is looking for alternatives to these products. The City of Berkeley is considering an ordinance that would ban maintenance uses of TCA.53
CONCLUSIONS
1. Some chemical emissions characteristic of routine casting operations, such as ammonia, formaldehyde, nitrogen dioxide, and phenol, may be present at levels which can be smelled by some persons but pose no health risk.54 The uncertainty is due to greater sensitivity of the human nose than of the analytic methods used; there is little uncertainty in the no risk” health conclusions for these chemicals.
2. The risk of inhalation of methylene chloride from PSC emissions cannot be judged in the absence of analytic data. See Recommendation 1, below.
3. The possibility of emission of irritant levels of fluorides cannot be judged, since this process has not been witnessed and no measurements have been made. Since fluorides have no odor, it is improbable that fluorides are a source of neighborhood effects which seem to be correlated with odors.
4. Information about the concentrations and the compounds of nickel and chromium present in stack emissions and/or air off-site is not adequate to judge the health hazard of these emissions. These elements and compounds do not have odors, so are not contributing to local odors. More sensitive analysis of these materials would be difficult, if not impossible.
RECOMMENDATIONS
1. Although the amount of methylene chloride used at PSC is not large on a daily basis, it is used in a spray formulation which would encourage dispersion. Methylene chloride should be analyzed in the air at three sites on or near the perimeter of the site and satisfactory to concerned neighbors in order to determine whether any of this carcinogen is migrating off-site. Since workers using this material would be at highest risk, two samples of air in the area where methylene chloride is used should be analyzed. If working area levels in excess of the lowered exposure level now under consideration by OSHA or off-site levels resulting in a cancer risk of greater than one in a million are found, then PSC should immediately seek a substitute mold release product and/or install ventilation and air filter equipment to protect workers and preclude off-site emissions.
2. PSC could be asked to provide quarterly reports on their progress in implementing the phaseout of TCA.
3. If there is concern about the possible irritant effects of fluoride, any future reports of eye irritation from PSC neighbors should be correlated with the times that fluoride containing products are being used for special castings. If there appears to be a correlation, fluoride should be analyzed in particulates from relevant stacks at a time when castings involving the use of, fluorides are being made.
1MSDSs must be sent with shipments of hazardous chemicals so that users will know what safety precautions to use.
2 Worthington, Guy, Project Manager, Aqua Terra Technology, studies performed for PSC, air samples taken in December 1989 and reported in 1990. December is part of the period in which air quality is worst in the Bay Area because air inversion layers trap pollutants near the ground.
3 ENSR Health Sciences, Pacific Steel Casting Company, Berkeley, California, A Screening Assessment of Ambient Air, 1989
4 1988 Clayton Environmental data included in ENSR report, op. cit.
5 Not tending to evaporate into the air.
6 Producer's identification number
7 Novolak resins are formed by the reaction of a phenol with an aldehyde in the presence of a catalyst and acid.
8 Mutagen = capable of causing inheritable genetic changes
9 RTECS On-Line, 1990. The mutagenic effects of hexamethylenetetramine are probably due to its metabolism to formaldehyde, see discussion of formaldehyde starting on page 6.
10 Sax, N. Irving and Lewis, Richard J., Sr., Dangerous Properties of Industrial Materials, 7th Ed., Van Nostrand Reinhold, 1989.
11 Odor detection information is not available for hexamethylenetetramine.
12 Environmental sources of PAHs include vehicle exhaust (particularly diesel vehicles), fireplace and barbecue smoke, and browned food. Some of these sources are avoidable, others are not.
13 State of California Air Resources Board, Indoor Air Quality and Personal Exposure, Briefing Paper, Research Division, 1987, page 22.
14 U. S. Department of Energy, Indoor Air Quality Environmental Information Handbook: Building System Characteristics, 1987, pp. 2-35
15 The sensitivity of a method for a particular chemical, or its detection threshold, varies with the sampling and analytical methods and with the types and amounts of other chemicals present.
16 The detection limit was different for different stacks due to differences in the chemical mixture in the different stack samples. In this case, it is possible that the 0.5 and 0.6 ppm readings were instrumental artifacts and the formaldehyde level was actually undetectable.
17 Department of Labor, Occupational Safety and Health Administration, 29 CFR Parts 1910 and 1926, Occupational Exposure to Formaldehyde; Final Rule, 52 Federal Register 46220, 4 December 1987.
18 The detection limit was lower in this study than in the stack emission study due to differences in methodology.
19 Persons with rare genetic conditions which affect the ability to repair damage to DNA molecules, such as xeroderma pigmentosum, are at greater risk than average when exposed to formaldehyde.
20 Most chemicals can be smelled at a lower concentration than the concentration at which the odor can be identified. The detection threshold or detection level for a chemical is the concentration at which the odor can first be smelled but may not be identifiable.
21 Amoore, John E., and Houtala, Earl, Journal of Applied Toxico fogy, 3:272-290, 1983.
22 State of California, Air Resources Board, op. cit., Table 1, Indoor Air Pollutants.
23 Bay Area Air Quality Management District, Air Quality Handbook, 1989-1990, page 20.
24 ENSR, op. cit.
25 American Conference of Governmental Industrial Hygienists (ACGIH), 7990-1991 Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices, ACGIH, 1990. Note that these occupational standards are established for healthy, working adults, and are not applicable to children, elderly persons, or to persons with medical conditions affecting their ability to work.
26 Sax, op. cit., page 2523.
27 Nitrogen dioxide has been extensively studied, so that a 10-fold safety factor is reasonable. Using a greater than ten-fold safety factor would result in prediction of acute effects at concentrations typically found in homes.
28 Becker, Walter E., Jr., et al., Fire Research on Cellular plastics: The Final Report of the Products Research Committee, National Bureau of Standards, 1980, page 96.
29 The odor of phenol is described by Verscheuren (op. cit.) as "medicinal, sickening sweet and acrid with a sharp and burning taste."
30 I am not aware of a consensus value for estimation of the carcinogenic risk of human exposure. Available evidence indicates that phenol is not a powerful carcinogen. Doses which induce cancer in animals are similar to the human lethal dose.
31 Small amounts of benzene may also be formed as a thermal degradation product of resins used in the casting process. Six tests for benzene in the emissions of two stacks found benzene to be undetectable in five samples with a detection threshold of 200 parts per billion (ppb) and 330 ppb in one sample. This maximum level of benzene would result in off-site benzene concentrations lower than it a gasoline station.
32 The odor threshold of ethylene dibromide is 25 ppm according to Gosselin, Robert E., et al., Clinical Toxicology of Commercial Products, 5th Ed., Williams & Wilkins, 1984, page 11-162. The sickening odor is not detectable at the concentration found in gasoline.
33 Teratogen = capable of causing birth defects.
34 Lack of data on human carcinogenicity usually means either or both that no suitably sized group of people has been identified that has been exposed to the chemical in question and no other known or suspected carcinogen or that funds have not been available for the necessary studies.
35 RTECS On-Line, 1990.
36 The asphaltenes have molecular weights in the range of 1,000 to 2,600; the resins are in the range of 370 to 500 and the oils are in the range of 290 to 630. Source: National Institute for Occupational Safety and Health (NLOSH), Criteria for a Recommended Standard: Occupational Exposure to Asphalt Fumes, NIOSH Document No. 78-106, 1977, as cited in Sittig, Marshall, Handbook of Toxic and Hazardous Chemicals and Carcinogens, 2nd Ed., Noyes, 1985, page 97.
37 Hydrocarbons are chemical compounds that contain carbon and hydrogen atoms. Saturated hydrocarbons have all single bonds. Unsaturated compounds have one or more double bonds between carbon atoms.
38 Computer search of Chemical Abstracts revealed no papers on the odor of asphalt.
39 Quenching oils are used in baths for carbon and alloy steels to remove heat from the steel more slowly and uniformly than results with water baths.
40 RTECS On-Line, 1990
41 RTECS On-Line, 1990
42 Verschueren, Karel, Handbook of Environmental Information on Organic Chemicals, 2nd Ed., Van Nostrand Reinhold, 1983
43 This assumes that an adult breathes 20 cubic meters of air a day, as prescribed by the California Department of Health Services in The California Site Mitigation Decision Tree, 1987. The figures for a child would be similar to those of an adult because although a child has a smaller lung volume than an adult, the child's respiratory rate is likely to be higher than that of an adult if the child is active.
44 Data on toxic doses by inhalation are not available in standard handbooks, suggesting that naphthalene is more toxic by the oral route than by inhalation.
45 Naphthalene is not known to be an allergic sensitizing agent.
46 Epoxy products vary in their sensitizing ability. 1 presume that the epoxy glues and paints on the consumer market are on the average less sensitizing than the industrial epoxies and that consumer exposure is lower than occupation exposure, therefore less likely to result in sensitization. Any use of two component epoxy products should be done under conditions of good ventilation and skin protection to minimize the risk of sensitization.
47 Some of the steels contain chromium to achieve desired physical and chemical properties. Chromium is present in PSC stack emissions at 0.00042 ppm (0.42 parts per billion) . Thecarcinogenicityofchromium ismainly associated with chromates. The emissions from PSC would be in the form of elemental chromium which is not a human or animal carcinogen according to IARC or in the form of chromium trioxide, not in the form of chromates. However, chromium trioxide is also carcinogenic and is water soluble. Typical East Bay concentrations of hexavalent chromium (the most carcinogenic fraction, including chromates and chromium trioxide, are 0.0002 parts per billion (Murchison, Gary, et al., Technical Support Document to Proposed Hexavalent Chromium Control Plan, State of California Air Resources Board, 1988). It is not known what portion of the total chromium in PSC stack emissions is due to hexavalent chromium. Nochromium measurements have been made off-site. Dilution by clean air, as the stack plume moves away from the emission point, would be expected to dilute the concentration down to typical East Bay levels by the time they reach the breathing zone of people off-site.
48 ENSR, op. cit.
49 Calculated on the basis of risk factor in California Department of Health Services, Toxic Air Pollutant Source Assessment Manual for California Air Pollution Control Districts and Applicants for Air Pollution Control District Permits, 1987, page 3.5-27.
50 RTECS On-Line, 1990.
51 Verschueren, op. cit., page 848.
52 RTECS On-Line, 1990.
53 Draft Ordinance Regulating the Sale, Use and Recycling of Products that Utilize Ozone-Depleting Compounds, undated.
54 People who do not smell the odors when others do should be informed that there is a wide natural variation in the human ability to perceive specific smells and that this may be accentuated by exposure experience which may raise or lower the detection level, depending on the chemical.
All Rights Reserved